pH Electrodes Informatio
 pH electrodes are analytical sensors for measuring potential of hydrogen (pH), the negative logarithm of the hydrogen ion activity in solution. The pH value of a substance is directly related to the ratio of the hydrogen ion [H+] and the hydroxyl ion [OH-] concentrations. This is one of the most common laboratory measurements because so many chemical processes are dependent on pH.
pH electrodes are analytical sensors for measuring potential of hydrogen (pH), the negative logarithm of the hydrogen ion activity in solution. The pH value of a substance is directly related to the ratio of the hydrogen ion [H+] and the hydroxyl ion [OH-] concentrations. This is one of the most common laboratory measurements because so many chemical processes are dependent on pH.
Production of pH electrodes. Video Credit: EndressHauserAG / CC BY-SA 4.0
pH electrodes work by producing an electrical potential between two liquids of different pH when they come into contact with opposite sides of a thin glass membrane, known as the pH element. This voltage potential is a function of the free acidity or free alkalinity of the solution. The pH element is permeable by H+ ions, and the pH electrode is filled with neutral solution, which by definition contains an equal number of H+ and OH- ions. The pH probe is then immersed in the H+ solution, and the glass membrane is permeated by the H+ ions which exert a positive potential on the sensing electrode. This potential difference is measured by a pH meter and converted to a pH output. When probe is immersed in an alkaline environment, the H+ ions migrate outside of the probe, leaving an excess of OH- ions within the probe, and a negative potential is sensed by the pH meter.
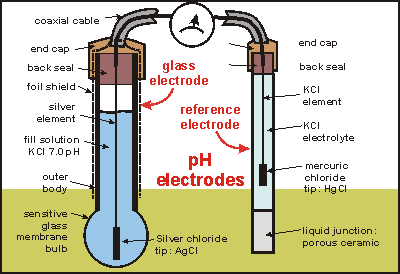 view full size image
view full size image
pH electrode parts. Image Credit: Sea Friends
pH electrodes are different than pH probes. pH probes are assemblies used for measuring pH and include an electrode, housing, and a temperature sensor.
Performance Specifications
With pH electrodes, there are three main performance specifications: pH range, pH accuracy, and response time.
- Wide-range pH electrodes function over the activity range 1 M H+ (pH 0) to 10-14 M H+ (pH 14).
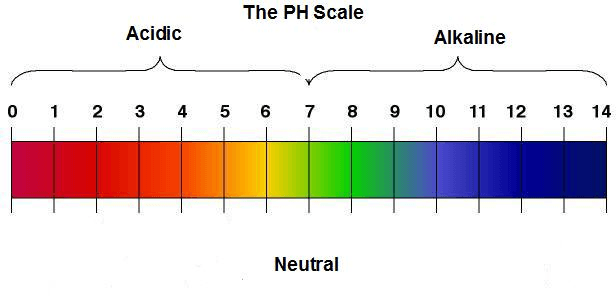 view full size image
view full size image
pH scale. Image Credit: Abundant Health Center
- Response time is usually expressed as a value such as 95% in 10 seconds.
For more information please read IEEE GlobalSpec's How to Select Oxidation Reduction (ORP) Electrodes.
Specifications
pH electrodes carry physical specifications for electrode configuration and reference solution options.
Single cells or electrode pairs require a separate reference electrode. This type of device is the best choice for colloidal suspensions, iodides in sample, and high-percentage solids in fluid.
Combination pH electrodes are also commonly available. These devices have two parts: the measuring electrode and the reference electrode.
pH Electrode Solution Options
In terms of reference solution options, reference electrodes are either refillable or sealed. The trade-off between the two types is the amount of maintenance versus length of product life. Refillable pH electrodes require more maintenance, but also last longer and typically have higher accuracies. Sealed pH electrodes do not require refilling, but have a limited life since the chemicals inside are used up and are not replaceable.
Junction Type
Junction type is an important physical specification to consider when selecting pH electrodes. There are two basic types:
Single junction- As their name suggests, single junction reference electrodes contain a single electrolyte. The electrolyte provides a constant level of the ion sensed by the reversible reference element and forms a low potential liquid junction with the sample solution. Single junction electrodes are the most common in the industry.
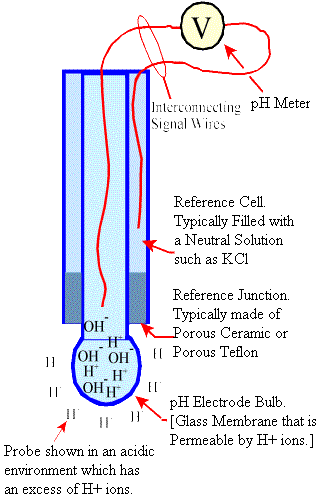
Single junction glass electrode. Image Credit: pHadjustment.com
Double junction- Double junction reference electrodes have an additional reference cell in front of the potassium chloride cell. This cell screens the sample from the potassium chloride reference cell. Typically, double junction cells have a longer life time because the second chemical barrier slows down the effects of electrode poisoning.
Body Material
There are two common choices for body material: glass, and epoxy or polymer.
Mounting Options
Mounting options are defined as handheld or portable, in-line (insertion), and flow-through (pipeline).
Handheld or portable configurations allow pH electrodes to be used in laboratories where an operator may need to test several different samples.
Insertion-style pH electrodes are often inserted into process piping through a tapped hole in a pipe or bulkhead.
Flow-through styles fit directly into the pipeline and become an integral part via some connection, such as flanges or other fittings.
pH Calibration and Maintenance
Video on selection and calibration of pH electrode. Video Credit: HachCompany / CC BY-SA 4.0
In order to get the best performance and life out of your pH electrode it's important to regularly clean and calibrate the electrode. The electrode should be stored in the recommended pH storage solution between measurements. The reference junction should not be allowed to dry out. If the electrode exhibits slow stabilization times, erratic readings, or has difficulties calibrating it may be time to clean the electrode. The best cleaning solution is one which interacts in a more selective way with the contamination (i.e. fats and oils should be cleaned with a non-ionic surfactant solution or methanol). After the cleaning solution, the electrode should be rinsed with deionized water and stored in the proper pH solution. The glass bulb can be cleaned by soaking in warm deionized water.
If the electrode is refillable, the electrode should be kept filled with the correct electrode filling solution. Check to ensure there is enough electrolytes inside the electrode before use.
Features
Typical features for pH electrodes include:
Temperature compensation- Temperature compensation is very important for an accurate pH measurement; however, it is not used for ORP measurement. Temperature does affect the reaction potentials for all chemicals but true ORP is the direct measurement of electrons moving during an oxidation-reduction reaction, regardless of temperature.
Built-in temperature sensing- Devices have a built-in calibrator, which can be used for testing and readjustment.
Submersible or water resistant design- Submersible ORP electrodes can be used in water testing applications.
Suited for food or sanitary applications- ORPs used in food or sanitary applications can be easily sanitized between uses to prevent contamination.
No comments:
Post a Comment