Non-catalyzed one-step synthesis of ammonia from atmospheric air and water
Tetsuya Haruyama *ab, Takamitsu Namise a, Naoya Shimoshimizu a, Shintaro Uemura a, Yoshiyuki Takatsuji a, Mutsuki Hino a, Ryota Yamasaki b, Toshiaki Kamachi c and Masahiro Kohno c
aDivision of Functional Interface Engineering, Department of Biological Functions and Engineering, Graduate, School of Life Science and Systems Engineering, Kyushu Institute of Technology, Kitakyushu Science and Research Park, Kitakyushu, Fukuoka, 808-0196, Japan. E-mail: haruyama@life.kyutech.ac.jp
bResearch center for Eco-fitting Technology, Kyushu Institute of Technology, Kitakyushu Science and Research Park, Kitakyushu, Fukuoka, 808-0196, Japan
cDepartment of Bioengineering, Tokyo Institute of Technology, Ookayama, Meguro-ku, Tokyo 152-8550, Japan
aDivision of Functional Interface Engineering, Department of Biological Functions and Engineering, Graduate, School of Life Science and Systems Engineering, Kyushu Institute of Technology, Kitakyushu Science and Research Park, Kitakyushu, Fukuoka, 808-0196, Japan. E-mail: haruyama@life.kyutech.ac.jp
bResearch center for Eco-fitting Technology, Kyushu Institute of Technology, Kitakyushu Science and Research Park, Kitakyushu, Fukuoka, 808-0196, Japan
cDepartment of Bioengineering, Tokyo Institute of Technology, Ookayama, Meguro-ku, Tokyo 152-8550, Japan
Received 8th June 2016 , Accepted 7th July 2016
First published on 7th July 2016
It is well known that ammonia is produced through a catalytic reaction at high temperature and pressure from pure nitrogen and hydrogen. This catalytic chemical process is a massive and high-energy-consuming process, but a very important one for nitrogen fixation. Here, we show a non-catalyzed one-step synthesis of ammonia from atmospheric air (nitrogen source) and water (hydrogen source), based on an interfacial reaction between the air plasma gas phase and the water phase, at 25 °C and atmospheric pressure. In the plasma/liquid interfacial reaction (P/L reaction), atomic nitrogen in both air plasma and nitrogen plasma first abstracts hydrogen from the water phase surface at the P/L interface, and then NH is produced without any catalyst. Transiently formed NH is reduced further at the water phase, affording NH3, which then dissolves in the water phase. The P/L reaction may provide an alternative solution that enables both energy conservation and CO2 emission reduction.
Introduction
Obtaining resources from atmospheric air (or other inexhaustible supplies of a desired compound) is a key objective in the science and engineering industries. The Haber–Bosch process is a pioneering achievement in this field, and is used to produce ammonia from pure hydrogen (H2) and pure nitrogen (N2) via a high temperature and pressure catalytic chemical process.1 However, such energy demanding processes are currently undesirable due to the ongoing energy burden.
There have been a number of major developments related to the chemical synthesis of ammonia with the aim of lowering the amount of energy consumed. For instance, a Fe3O4-catalyst has been used as the catalyst in the Haber–Bosch process; furthermore, various groups have been studying ways of developing new catalysts and improving the process. These efforts have enhanced the process to some degree, as some of the improved catalysts, such as the Ru-loaded electride catalyst, are effective in reducing the reaction temperature.2
Other approaches have also been employed for synthesizing ammonia, including using a plasma process. Gómez-Ramírez et al. synthesized ammonia from pure N2 and H2 under a plasma gas atmosphere while using ferroelectric material beads as the catalyst.3 In this case, the catalytic effect of the ferroelectric increased the reaction rate. In another study, ammonia was produced from pure N2 and ethanol, which was used as the hydrogen source.4 In this reaction system, pure N2 plasma gas was blown into the liquid phase (plasma bubbling system). When the plasma gas was bubbled into water, only a small amount of ammonia was produced, with the liquid phase containing NO3−. On the other hand, when the plasma gas was bubbled into a 20% (v/v) ethanol solution, a significant amount of ammonia was produced in the liquid phase, with nitrate forming as a by-product. Thus, in this reaction system, ethanol acted as the sacrificial reagent (i.e. as the hydrogen source) for the synthesis of ammonia.
Procurement of raw materials is also problematic since pure hydrogen is usually produced from crude oil or natural gas. Pure nitrogen preparation also requires energy; in addition, both pure H2 and N2 must be transported to the ammonia production site. Increasing the number of steps in processing and transportation inflates both energy consumption and carbon dioxide emission. Hence, continued and persistent technical trials are required for energy conservation and reduction of carbon dioxide emission.
In Japan in particular, following the Great East Japan Earthquake in March 2011 and the subsequent power source crisis, energy conservation has become increasingly important. People who are aware of the crisis exercise energy conservation and reduction of carbon dioxide emission.
Many different improvement technologies have been investigated to improve the Haber–Bosch ammonia production process. However, all of these improved technologies still require pure nitrogen and pure hydrogen as the chemical precursors as well as a catalyst or chemical sacrificial reagent. They also involve heat energy and/or high pressures.
If a non-catalytic hydrogen adduct reaction could be performed at the standard temperature and atmospheric pressure, it would help resolve the problem of energy burden.
We previously discovered a typical interfacial non-catalytic reaction between a plasma gas phase and a water phase, and reported the quantitative production of hydroxyl radicals (˙OH) and singlet oxygen (1O2).5 At the oxygen plasma/water interface, atomic oxygen, which is produced from the ultraviolet irradiation of oxygen plasma, abstracts a hydrogen atom from the surface of the water phase.5 This donation of a hydrogen atom to atomic oxygen by the interfacial reaction between the plasma gas phase and the water phase is key in the formation of the hydroxyl radical (˙OH). The authors proposed the reaction mechanism of hydroxyl radical (˙OH) formation as follows. The abstraction of a hydrogen atom from H2O is generally difficult because of the high binding energy (∼499 kJ mol−1 in H2O, cf. ∼430 kJ mol−1 in ˙OH). Although hydrogen bonding on the surface is stronger than in the bulk, some hydrogen bonding is lost, and so a more reactive environment can be obtained on the water surface.6 The surface of the water phase may therefore act as a reaction locus to donate a hydrogen atom from H2O to the atomic gas molecules of the plasma phase.
In the present study, a non-catalytic one-step synthesis of ammonia from atmospheric air (mixed gas with 78% N2 + 21% O2 + traces of other gases) and water (H2O) is reported, based on an interfacial reaction between an air plasma gas phase and a water phase under UV irradiation at the standard temperature and pressure (25 °C, atmospheric pressure).
Materials and methods
Interfacial reaction locus between a plasma phase (gas phase) and a water phase (liquid phase) – the plasma–liquid interface reaction (P/L reaction)
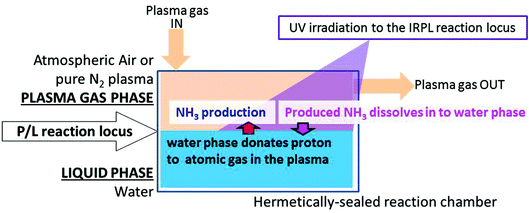
A schematic outline of the interfacial reaction locus is shown in Fig. 1. The interfacial reaction locus (i.e., the locus of the P/L reaction) is an interface between a plasma gas phase and a water phase, and in this case involves the irradiation of the interface with UV light. The reactor was originally constructed using a retrofitted Radical Vapor Reactor (Ebara Jitsugyo Co., Ginza, Tokyo). The reactor produced plasma gas by electrodischarge (6 kV) using either pure nitrogen gas (99.95%) or atmospheric air (taken from a compressed air cylinder), which was introduced at 4 L min−1 to the P/L reaction locus. Pure deionized water was used for the pure water phase (specific electric resistance of deionized water ≥18.2 MΩ cm−1), which was contained in round flat glass dishes of various areas (i.e. 5.75, 13.53, 27.34, 38.48, 62.21, 76.2, 105.68, and 167.42 cm2) placed inside the reactor. In an experiment with heavy water (D2O) for the water phase in a P/L reaction, D2O (Cambridge Isotope Laboratories, Inc., UK) was contained in round flat glass dishes of area 167.42 cm2.
UV light sources of 185 nm (0.16 W × 2 lamps) and 254 nm (1.6 W × 2 lamps) were used. All P/L reactions were performed at ambient temperature (25 °C) and atmospheric pressure unless otherwise indicated in the figure legend.
Quantitative analysis of produced ammonia and by-products
Spectrophotometric determination of the ammonia content has previously been performed using the indophenol blue reaction.7 In the present study, an indophenol blue reaction assay kit (WAKO Pure Chemical Industries Ltd, Japan) was employed for ammonia detection.
The reaction by-products (NOx substances) in the gas phase were assayed using a KITAGAWA gas detector tube (Komyo Rikagaku Kogyo K.K., Japan).
Nitrite ions in the water phase were assayed by ion-chromatography (Prominence HIC-SP, SHIMADZU Co. Ltd Japan) with a Shim-pack IC-SA3 column (SHIMADZU).
Results and discussion
A schematic representation of the interfacial reaction locus (phase interface reaction system) is illustrated in Fig. 1. The locus of the plasma/liquid interfacial reaction (P/L reaction) is essentially the interface between the plasma gas phase and the water phase, which is the locus of hydrogen abstraction from the water phase surface by atomic nitrogen in the plasma gas phase. The P/L reaction gives ammonia from atmospheric air and water alone in a one-step reaction.
The quantity of ammonia produced is dependent on the area of the P/L reaction locus irradiated with UV light, as shown in Fig. 2. As can be seen in the figure, the produced ammonia, which was dissolved in the water phase, increased with an increase in reaction locus area. In the absence of UV irradiation, the amount of ammonia production decreased (data not shown). It was known that irradiated UV energy promotes dissociation of atoms in plasma gas.5 The UV irradiation at the reaction locus may promote atomic nitrogen in plasma gas.
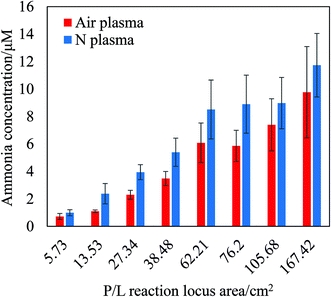 | ||
| Fig. 2 Effect of the interface area on ammonia production in the P/L reaction. Two different plasma gases (atmospheric air and 99.95% N2 gas) were employed and compared. | ||
As shown in Fig. 2, the surface area dependency of the P/L reaction locus (water surface area) confirmed that the P/L reaction is a typical interfacial reaction (heterogeneous reaction) that takes place between the air/plasma gas phase and the water phase. Ammonia production was then performed under pure nitrogen or in atmospheric air. In air, ammonia production was ∼25% lower than in pure nitrogen, likely due to atmospheric air containing only 78% N2. Furthermore, this observation suggests that contamination with oxygen does not affect ammonia production in the P/L reaction. Importantly, this reaction can produce ammonia directly from atmospheric air and water, whereas the Haber–Bosch process requires pure hydrogen (H2) and pure nitrogen (N2).
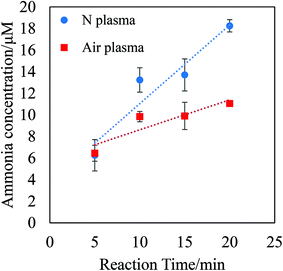
Fig. 3 shows the amount of ammonia produced in the water phase during the P/L reaction between 0 min to 20 min. In these experiments, atmospheric air plasma or pure nitrogen plasma were employed, with an increase in ammonia production being obtained for both systems with time. As shown in Fig. 3, the amount of produced ammonia at each time was comparable for both the atmospheric air plasma system and the pure nitrogen plasma system, while taking into consideration the ∼25% drop in production for the air system. Time dependence of the ammonia content is probably affected by ammonia dissociation by UV. Ammonia has been produced at the P/L reaction locus with UV irradiation. But irradiated UV energy also dissociates part of ammonia. For the practical application of the P/L reaction, produced ammonia has to be tapped off from the reaction chamber in future systemization.
In the P/L reaction employing atmospheric air and pure water, the production of by-products such as NO or NO2 was also possible, as atmospheric air contains approximately 78% N2, 21% O2, 0.1% Ar, and 0.03% CO2 along with other minor components.
Table 1 shows the amounts of ammonia and by-products produced during the P/L reaction at the interface between the water phase and the nitrogen plasma gas phase with or without UV irradiation. An important property of the UV irradiation process is that it had a positive effect on the amount of ammonia produced, with twice as much ammonia being synthesized when UV irradiation was performed. This result strongly suggests that the UV irradiation provided additional reaction energy for the extraction of hydrogen from the surface of the water phase by the atomic nitrogen in the plasma phase.
Table 1 Products formed by the P/L reaction. The P/L reaction was performed at the interface of a pure nitrogen plasma gas phase and a water phase
The P/L reaction exhibits a number of advantages compared to other plasma chemical processes. Most plasma chemical processes, such as the plasma bubbling chemical process,4 are based on homogeneous reactions involving the bubbling of plasma gas in solution. In this case, ethanol is required as a sacrificial reagent (i.e. as hydrogen source) for amplifying the ammonia synthesis. However, the P/L reaction proposed in this study is a heterogeneous interfacial reaction between a plasma gas phase and a water phase. UV irradiation (i.e. deliverance of UV energy) can be performed on the plasma gas phase effectively. It is critical that UV light is irradiated at the locus of the interfacial reaction. In the case of a similar interface between hydrophilic and hydrophobic phases, site-specific UV light absorption has been observed.8 This suggests that the surface of the water phase (i.e., the P/L reaction locus) absorbs the energy from UV irradiation. As described by Marx in a simulation study, the H atoms in the H2O molecule are oriented outwardly on the surface of the water phase, owing to the high surface tension.9 The UV energy probably increases the rate of the reaction for the extraction of hydrogen from the surface of the water phase by atomic nitrogen in the plasma gas phase.
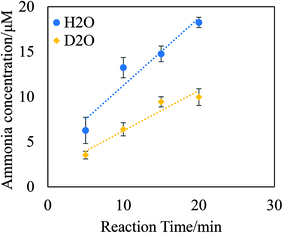
The P/L reaction was also performed using heavy water (D2O) for the liquid phase, as shown in Fig. 4. In the case of the P/L reaction with H2O, 11.7 μM of ammonia was produced (area of the P/L reaction locus = 167.4 cm2, flow rate of plasma gas = 4 L min−1, reaction time = 10 min). However, in the case of the P/L reaction with D2O, the amount of ammonia produced was less than half of that produced when H2O was used, as shown in Fig. 4. The reaction-time dependence of the amount of ammonia produced showed that the ammonia production rate in the case of D2O was less than half of that in the case of H2O. This was because of the kinetic isotope effects of deuterium. This result indicates that the rate-limiting step of the P/L reaction is hydrogen extraction from the surface of the water phase. The difference of the ammonia production ratio is expressed in terms of the difference of mass between deuterium and hydrogen. The results clearly suggested that the hydrogen in the produced ammonia was abstracted from the surface of the water phase. This is an important fact that shows that the non-catalytic hydrogen adduct reaction is progressed by the P/L reaction.
The results discussed above show that the P/L reaction is a heterogeneous reaction at the interface of a plasma gas phase and a water phase. As the P/L reaction requires only atmospheric air and water as raw materials, these two substances are directly converted into ammonia via the one-step reaction. In addition to the significant advantage of allowing one-step ammonia production that can be initiated using atmospheric air and water, the P/L reaction has a remarkable energetic yield. The P/L reaction system requires electrical energy only for the electric discharger and the UV lamp. The total electric power consumed is less than 5 W h (actual measured value). For a P/L reaction locus area of 1 m2 and a reaction time of 10 min, 3.08 mg m−2 of ammonia was produced in the water phase. Thus, the current P/L reaction system produced 3.7 mg W−1 h−1 m−2 of ammonia even though it is not optimized.
In the P/L reaction, the water surface clearly acts as a proton-donor to the gaseous atomic nitrogen species in the plasma phase. Indeed, we reported the observation of H-donation in a P/L reaction in our previous study. It was revealed that the water surface acts as an H-donating reaction locus to the attached O plasma phase (produced by the electro-discharge of pure oxygen) with UV irradiation at the interface between the two phases.5 It is known that the electro-discharge of oxygen produces high concentrations of ozone. Indeed, in the ozone layer, UV-irradiated ozone produces atomic oxygen (O) and dioxygen (O2),5,10 with the reaction producing atomic oxygen being similar to the reaction of ammonia outlined herein.
In the production of ammonia through the P/L reaction, atomic nitrogen gas was present in the discharged air (nitrogen) plasma. The bond dissociation energy of nitrogen is 9.1 eV (8.7 × 105 J mol−1), and its ionization energy is 14.53 eV (1.39 × 106 J mol−1). In the present discharging experiments, a 6 kV silent electric discharge voltage was applied. With 50% efficiency in the discharge process, this converts to approximately 3 kV. The silent electric discharge of nitrogen therefore has sufficient energy to produce atomic nitrogen in the resulting plasma gas. A number of research groups have developed experimental and theoretical studies on N2 plasma using optical emission spectroscopy, considering various reactions (e.g. N2 + e− → N* + N + e−) based on the collisional relaxation of the ground state molecular N2 to deduce the nitrogen atom density.11,12 The reactivity of atomic nitrogen was also investigated through the azotization of carbon nanotubes.13
The enthalpy change for the production of ammonia from N2(g) and H2(g) is −46.1 kJ mol−1, indicating that this is an exothermic reaction, but with a very low reaction rate at room temperature. This is because nitrogen gas is very unreactive due to its strong triple bonds (bond dissociation energy = 8.7 × 105 J mol−1, 9.1 eV). Thus, to reduce the activation energy required for N2 bond dissociation, the Haber–Bosch process uses a heterogeneous catalyst to absorb N2 and promote the reaction. In the P/L reaction, nitrogen bond dissociation occurs in the discharged nitrogen plasma, and the nitrogen atoms then react with hydrogen at the water surface (in the P/L reaction locus) to form ammonia. The produced ammonia molecules then immediately dissolved in the water phase. The H–OH dissociation energy required by this process is 492 kJ mol−1, which is partially compensated for by the H–NH2 formation energy (460 kJ mol−1). Thus, the additional energy input required for the reaction to take place is ∼32 kJ mol−1, which can be achieved at ambient temperature, and in particular on the water surface, where reactive water molecules are present. With irradiation of the plasma/water interface using UV light at maximum wavelengths of 185 nm (646.6 kJ mol−1) and 254 nm (421 kJ mol−1), energy is provided to the water/plasma interface. In our experiment, it was observed that the ammonia production ratio decreased when a surface tension depressant (e.g., ethanol) was added to the water phase. These suggest that the water phase surface may act as a hydrogen-abstraction reaction locus for atomic nitrogen in the plasma gas phase in a similar manner to heterogeneous catalyst surfaces.
Based on the above considerations, we propose that ammonia formation at the P/L interface reaction proceeds as follows:
The produced ammonia is dissolved and it protonates in water through a known process to NH4+.14 As described in the results, the P/L reaction produces ammonia from atmospheric air and water in a very simple interfacial reaction. This is simply the foundation of a novel reaction system which requires further refinement, but has potential.
Based on the results described above, we can conclude that the P/L reaction process is capable of performing nitrogen fixation (ammonia production) through a very simple reaction system that requires scant electricity for electric discharge. Thus, the P/L reaction produces ammonia with low-energy consumption. In addition, an ordinary-pressured reactor can be fabricated at a moderate cost. The materials for P/L ammonia production are ubiquitous materials (atmospheric air and water) and can be obtained almost anywhere on the globe. This means that, material transport is not required for P/L ammonia production; therefore CO2 emissions from material preparation and transport can be reduced. The P/L reaction can be employed for on-site production at the ammonia-consuming region. It goes without saying that the demand for ammonia for fertilizer production, as a reductant in denitration processes, in synthetic fabric production, and as a hydrogen carrier will increase in the near future.
A practical advantage is that the P/L reaction produces ammonia through a one-step reaction only from atmospheric air (N source) and water (H source) at ordinary temperatures and pressures without any catalyst and sacrificial reagent. The P/L reaction should aid in the development of a flow system that can be used to produce ammonia on a practical large scale.
The new P/L reaction may provide an alternative chemical process that allows both energy conservation and reduction of carbon dioxide emission.
Acknowledgements
This research study has been partially supported by a research grant from the New Energy and Industrial Technology Development Organization (NEDO), Japan.References
- V. Smil, Detonator of the population explosion, Nature, 1999, 400, 415 CrossRef CAS.
- S. Kanbara, M. Kitano, Y. Inoue, T. Yokoyama, M. Hara and H. Hosono, Mechanism Switching of Ammonia Synthesis Over Ru-loaded Electride Catalyst at Metal-Insulator Transition, J. Am. Chem. Soc., 2015, 137, 14517–14524 CrossRef CAS PubMed.
- A. Gómez-Ramírez, J. Cotrino, R. M. Lambert and A. R. González-Elipe, Efficient synthesis of ammonia from N2 and H2 alone in a ferroelectric packed-bed DBD reactor, Plasma Sources Sci. Technol., 2015, 24, 065011 CrossRef.
- Y. Kubota, K. Koga, M. Ohno and T. Hara, Synthesis of ammonia through direct chemical reaction between an atmospheric nitrogen plasma jet and a liquid, J. Plasma Fusion Res., 2010, 5, 042 CrossRef.
- K. Matsuo and T. Haruyama, et al., Dispersed-phase interfaces between mist water particles and oxygen plasma efficiently produce singlet oxygen (1O2) and hydroxyl radical (·OH), Electrochemistry, 2015, 83(9), 721–724 CrossRef CAS.
- W. Kuo and C. J. Mundy, An ab initio molecular dynamics study of the aqueous liquid-vapor interface, Science, 2004, 303, 658–660 CrossRef PubMed.
- W. T. Bolleter, C. J. Bushman and P. W. Tidwell, Spectrophotometric Determination of Ammonia as Indophenol, Anal. Chem., 1961, 33(4), 592–594 CrossRef CAS.
- B.-h. Chai, J.-M. Zheng, Q. Zhao and G. H. Pollack, Spectroscopic Studies of Solutes in Aqueous Solution, J. Phys. Chem. A, 2008,112(11), 2242–2247 CrossRef CAS PubMed.
- D. Marx, Throwing Tetrahedral Dice, Science, 2004, 303(1), 634–636 CrossRef CAS PubMed.
- T. Tasaki, et al., Degradation of methyl orange using short-wavelength UV irradiation with oxygen microbubbles, J. Hazard. Mater., 2009,162, 1103–1110 CrossRef CAS PubMed.
- H. Akatsuka, Progresses in Experimental Study of N2 Plasma Diagnostics by Optical Emission Spectroscopy, in Chemical Kinetics, ed. V. Patel, InTech - Open Access Publisher, 2012, ch. 13, pp. 283–308 Search PubMed.
- Y. Ichikawa, et al., Actinometry Measurement of Dissociation Degree of Nitrogen and Oxygen in N2–O2 Microwave Discharge Plasma,Jpn. J. Appl. Phys., 2010, 49, 1060101 Search PubMed.
- Y. Zheng, Y. Jiao, L. Ge, M. Jaroniec and S.-Z. Qiao, Two-Step Boron and Nitrogen Doping in Graphene for Enhanced Synergistic Catalysis, Angew. Chem., Int. Ed., 2013, 125, 3192–3198 CrossRef.
- A. Babara Lechner, K. Youngsoon, P. J. Feiblman, G. Henkelman, H. Kang and M. Salmeron, Solvation and reaction of ammonia in molecularly thin waster films, J. Phys. Chem. C, 2015, 119, 23052–23058 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |



No comments:
Post a Comment